IS IT TIME TO THINK DIFFERENTLY ABOUT YOUR PATIENTS WITH REFRACTORY GENERALIZED MG?
Start by watching this short video about the pathophysiology of MG disease
(Viewing time: <3 minutes)
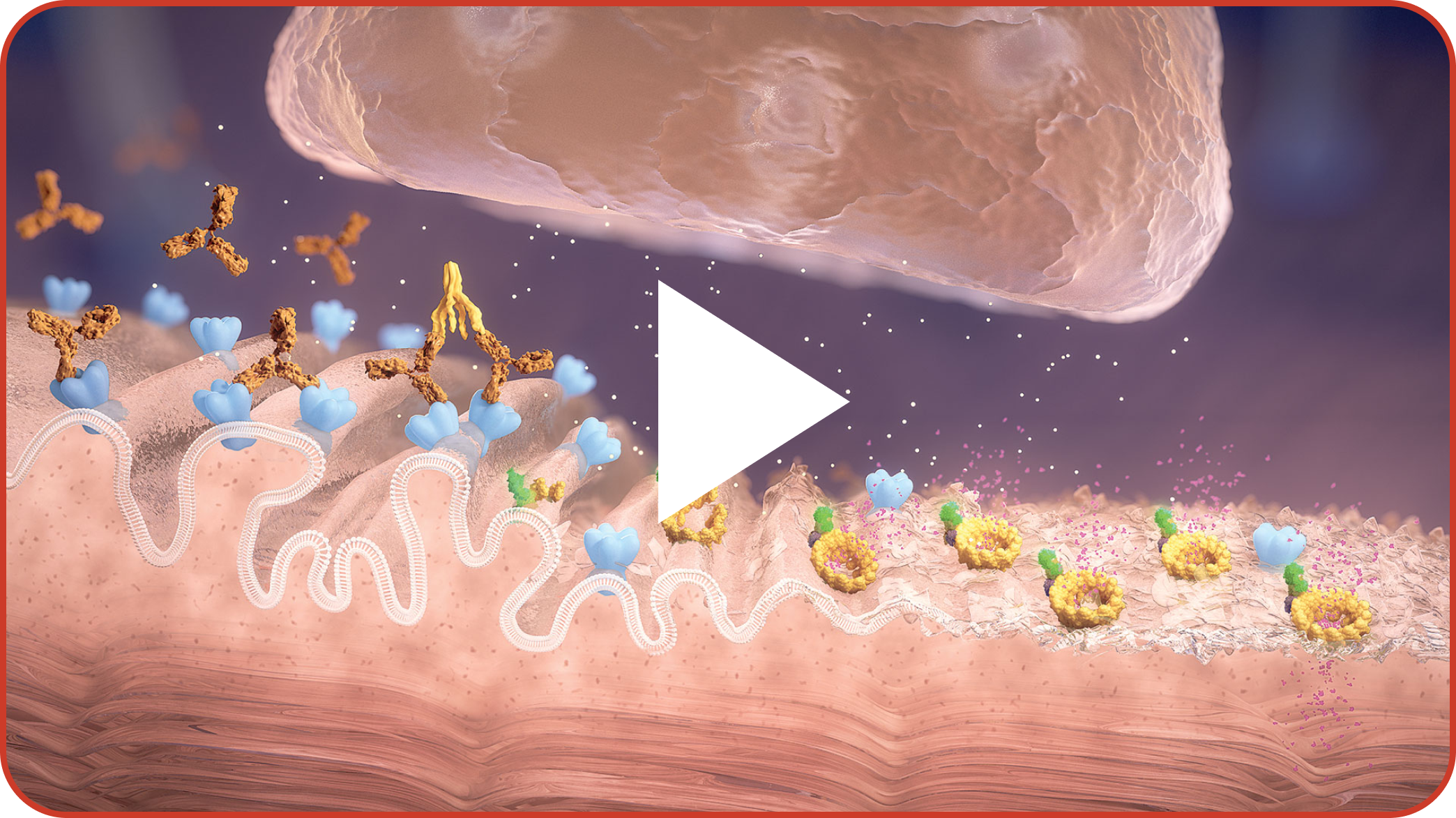
Start by watching this short video about the pathophysiology of MG disease
(Viewing time: <3 minutes)
How do you assess persistent symptoms in your patients with REFRACTORY GENERALIZED MG (gMG)?
In a prospective study, patients with refractory gMG experienced ongoing symptoms as measured by the MG-ADL, with a mean score (SD) of 10.2 (2.8)1,a,b
The QMG score has been shown to correlate well with the MG-ADL score.
Are your patients with refractory gMG experiencing declines in their quality of life?
In a retrospective analysis, patients with refractory MG experienced an impact on their health-related quality of life as measured by the MG-QoL15, with an average score (SD) of 31.4 (11.1)1,e,f,g

Daily burdens of refractory gMG that is assessed by the MG-QoL151
Frustrated by MG
Difficulty speaking
Trouble using eyes
Trouble driving
Trouble eating
Depressed about MG
Limited social activity
Trouble walking
Limited ability to enjoy hobbies/fun
Trouble getting around public places
Trouble meeting family needs
Feel overwhelmed by MG
Have to make plans around MG
Trouble with personal grooming
Occupational skills/job negatively affected
How many of your patients with refractory gMG experience exacerbations or myasthenic crises?
In a retrospective analysis, patients with refractory gMG who experienced a major event during a 1-year period3,h,i,j
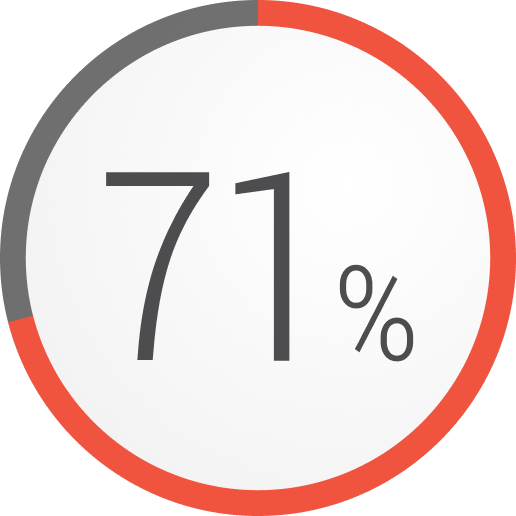
Exacerbations
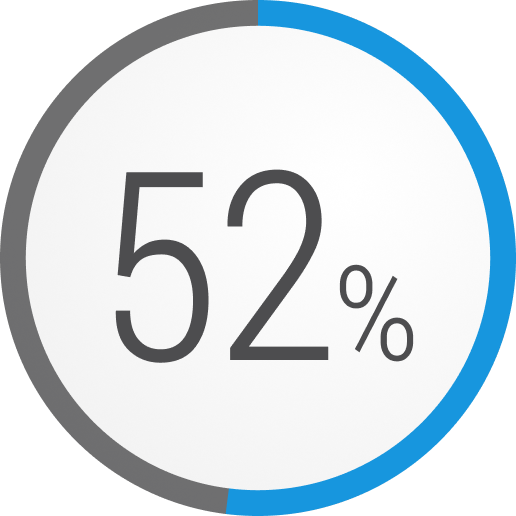
Hospitalizations
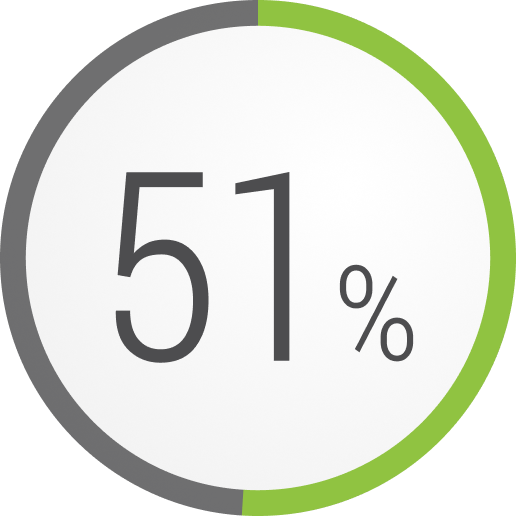
Emergency room visits
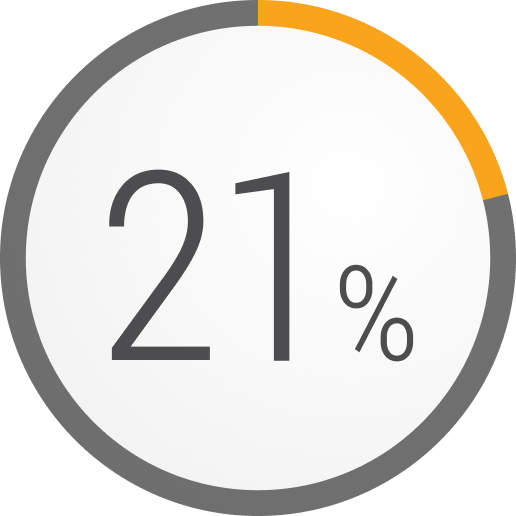
Myasthenic crisis


For your patients with refractory gMG:
Perform a Quantitative Myasthenia Gravis (QMG) ASSESSMENT today
-
Based upon the study-defined criteria of refractory gMG.
-
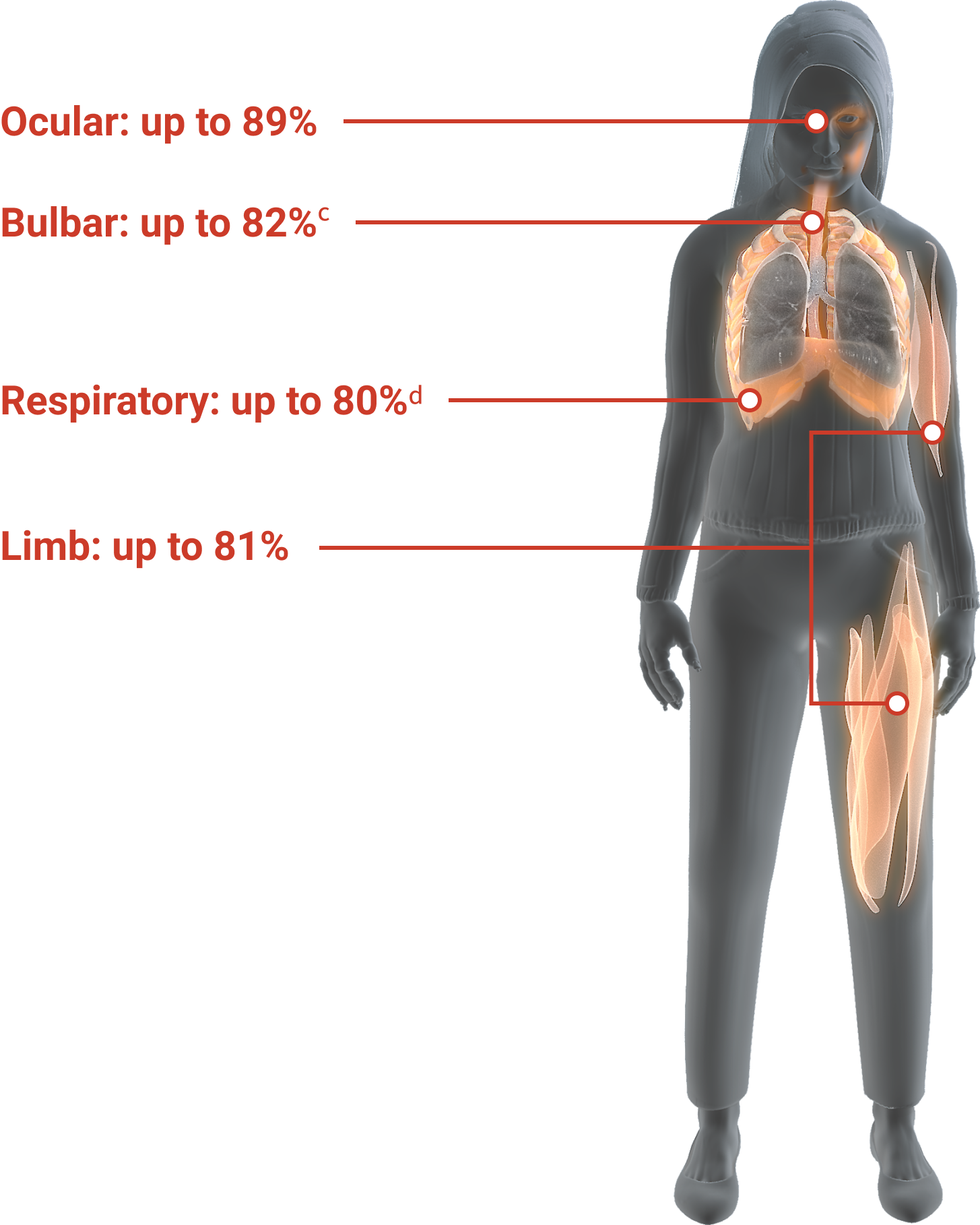
Data from a randomized, double-blind, placebo-controlled, multicentre trial of 125 patients with AChRAb+ refractory gMG. Patients were required to have previously failed treatment for ≥1 year with ≥2 immunosuppressive therapies or failed treatment with ≥1 immunosuppressive therapy and required chronic plasma exchange or IVIg, and had an MG-ADL total score ≥6 at study entry. Percentages reflect the highest percent manifestations within each symptom domain at baseline. Bulbar (difficulty chewing): 103 of 125 patients; respiratory (shortness of breath): 100 of 125 patients; ocular (ptosis): 111 of 125 patients; limb (impairment of ability to arise from a chair): 101 of 125 patients. All scores based on the 8-item MG-ADL assessment, with each item scored from zero (normal or none) to 3.1,2
-
Study excluded patients who required a gastric tube.1
-
Study excluded patients who were ventilator-dependent.1
-
Based upon the study-defined criteria of refractory gMG.
-
The MG-QoL15 is a 15-item questionnaire designed to assess how MG impacts various aspects of patient quality of life. Each of the 15 items is scored from zero (normal) to 4, with a maximum total score of 60.1
-
Data from the MGFA Myasthenia Gravis Patient Registry Enrollment Survey of 773 patients with MG, 56 of whom were refractory to treatment. Refractory patients reported worse scores across all 15 items of the MG-QoL15 and had significantly worse overall scores than non-refractory patients (total mean score [SD], 31.4 [11.1] vs 20.8 [15.0]; p<.01).1
-
Based upon the study-defined criteria of refractory gMG.
-
Data from an analysis of 403 patients with refractory gMG, 3811 patients with non-refractory gMG, and 403 healthy controls (or similar) from a US national administrative claims database.3
-
Note that hospitalizations and emergency room visits are not necessarily linked to refractory gMG.1
references:
-
Data on file, Alexion Pharmaceuticals.
-
ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT01997229. Updated March 1, 2017. Accessed May 20, 2017.
-
Engel-Nitz NM, Boscoe AM, Wolbeck R, Johnson J, Silvestri N [ICNMD abstract 146]. J Neuromusc Dis. 2016;3(suppl 1):S198-S199. doi:10.3233/JND-160001. Poster presented at: 14th International Congress on Neuromuscular Diseases; July 5-9, 2016; Toronto, Canada.